FDA Approves New Treatment for Significant Hair Loss
April 12, 2023
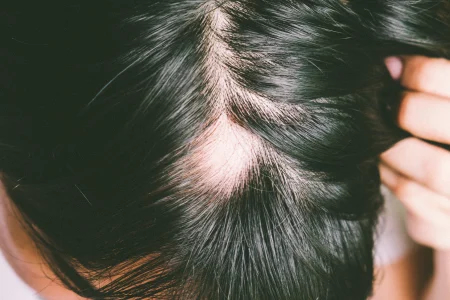
The FDA has recently approved Baricitinib (Olumiant; Eli Lilly and Company), an oral Janus kinase (JAK) inhibitor, to treat patients with alopecia areata (significant hair loss) (AA). AA is an autoimmune disorder that makes hair fall out as the body attacks the hair follicles. This disorder affects more than 300,000 people in the U.S. each year. This is the first FDA approval of a systemic treatment for AA.
Clinical trials have shown baricitinib to be safe and effective in the treatment of severe alopecia. It is anticipated that baricitinib will help fulfill a significant unmet need for patients with severe alopecia areata. The data behind the approval comes from 2 trials which were randomized, double-blind, placebo-controlled trials containing patients who had at least 50% scalp hair loss as measured by the Severity of Alopecia Tool for more than 6 months. Those in the trial were placed into 1 of 3 arms—placebo, 2 mgs of baricitinib, or 4 mgs baricitinib—every day. The key primary endpoint of the trials was the proportion of patients who achieved at least 80% scalp hair coverage at week 36.
In the first trial it was found that 22% of the 184 patients who received 2 milligrams of baricitinib achieved adequate scalp hair coverage compared to 35% of the 281 patients who received 4 milligrams of baricitinib. Five percent of the 189 patients who received a placebo.
In the second trial it was found that 17% of the 156 patients who received 2 milligrams of baricitinib achieved adequate scalp hair coverage compared to 32% of the 234 patients who received 4 milligrams of baricitinib. Three percent of the 156 patients who received a placebo.
Common adverse events in the use of baricitinib included upper respiratory tract infections, headache, acne, high cholesterol (hyperlipidemia), increase of an enzyme called creatinine phosphokinase, urinary tract infection,liver enzyme elevations, inflammation of hair follicles (folliculitis), fatigue, lower respiratory tract infections, nausea, genital yeast infections (Candida infections), anemia, low number of certain types of white blood cells (neutropenia), abdominal pain, shingles (herpes zoster) and weight increase.
Baricitinib (Olumiant) was originally approved in 2018. It is approved as a treatment for certain adult patients with moderately to severely active rheumatoid arthritis. Olumiant is also approved for the treatment of COVID-19 in certain hospitalized adults.
Dr. Moore and her dermatology team provide a number for solutions for hair loss. Dr. Moore also participates in ongoing research on hair loss treatment for alopecia areata. Call or text us at 817-755-5542 for more information or go to https://www.arlingtonskindoctor.com/clinical-trials/ to see if you qualify for free medication and free treatment for alopecia areata.
Source: FDA website, 2022; DERMATOLOGY TIMES, June 2022







