New topical study for ages 2-12 years old. This study focuses on the scalp and body.
While severe psoriasis affects the skin, causing it to itch and burn, it can also have an emotional impact on adolescents, as well as their parents.
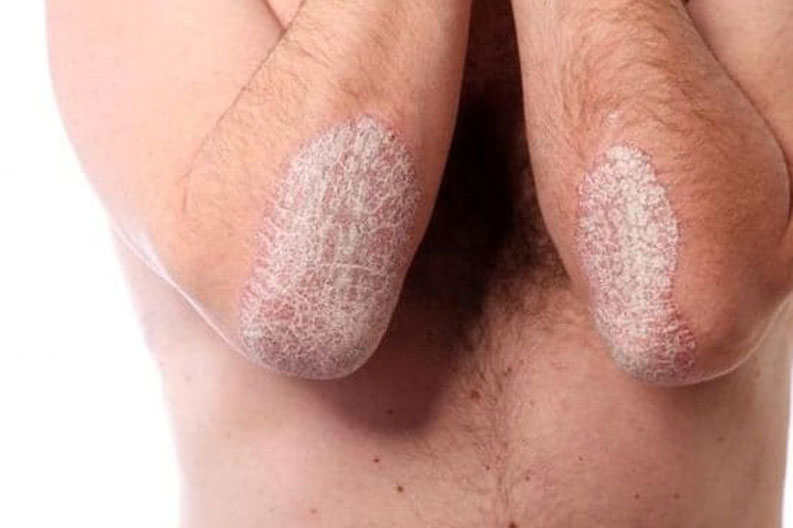
Because of the raised, red, scaly patches on their skin, many adolescents feel ashamed or embarrassed by their condition. These emotions can cause them to not want to hang out with friends, attend school functions, or participate in extracurricular activities.
For parents watching these physical and emotional effects, it’s difficult when other treatments have not been successful in managing their child’s psoriasis.
Because many adolescents and their families struggle with psoriasis, this research study is being conducted to evaluate an investigational drug for severe psoriasis. In this study, researchers want to learn more about the safety and effectiveness of the investigational drug.
The investigational drug has not been approved by any regulatory agency for the treatment of children and adolescents with this condition. It is only available to children and adolescents in research studies like this one.
The results of this study will provide more information about the investigational drug and whether it could one day be approved for children and adolescents with severe plaque psoriasis.
Who is eligible to participate in this study?
To be eligible for this study, patients must:
- Be 6 to 12 years of age
- Have been diagnosed with chronic plaque psoriasis
All study-related visits, tests, and drugs will be provided at no cost. In addition, compensation for study-related travel may be provided.