CLASSIC LIST
Our experience with Gott Marketing has been nothing short of exceptional. As a specialized marketing agency catering to the building products and construction industry, we initially approached Gott Marketing with specific industry-related concerns. However, our collaboration took an unexpected turn when we needed a new website for Arlington Skin Doctor.
Despite their primary focus on the construction sector, the team at Gott Marketing went above and beyond to accommodate our needs. They not only accepted the challenge of revamping our website but also demonstrated their versatility in tailoring their services to different industries.
From the initial consultation to the final rollout, the Gott Marketing team exhibited professionalism, creativity, and an in-depth understanding of our unique requirements. The result was a website that not only met but exceeded our expectations. The user-friendly design, seamless navigation, and visually appealing layout have significantly enhanced our online presence.
Gott Marketing’s commitment to delivering high-quality solutions and their willingness to adapt to diverse industries showcase their dedication to client satisfaction. We appreciate their flexibility, expertise, and the collaborative spirit that made our website redesign project a success.
For any business, regardless of industry, looking for a marketing agency that combines specialization with adaptability, Gott Marketing is undoubtedly a top choice. Their ability to pivot and deliver outstanding results sets them apart in the competitive landscape of marketing agencies.
Thank you, Gott Marketing, for your outstanding work and commitment to ensuring our online presence aligns with our brand values.
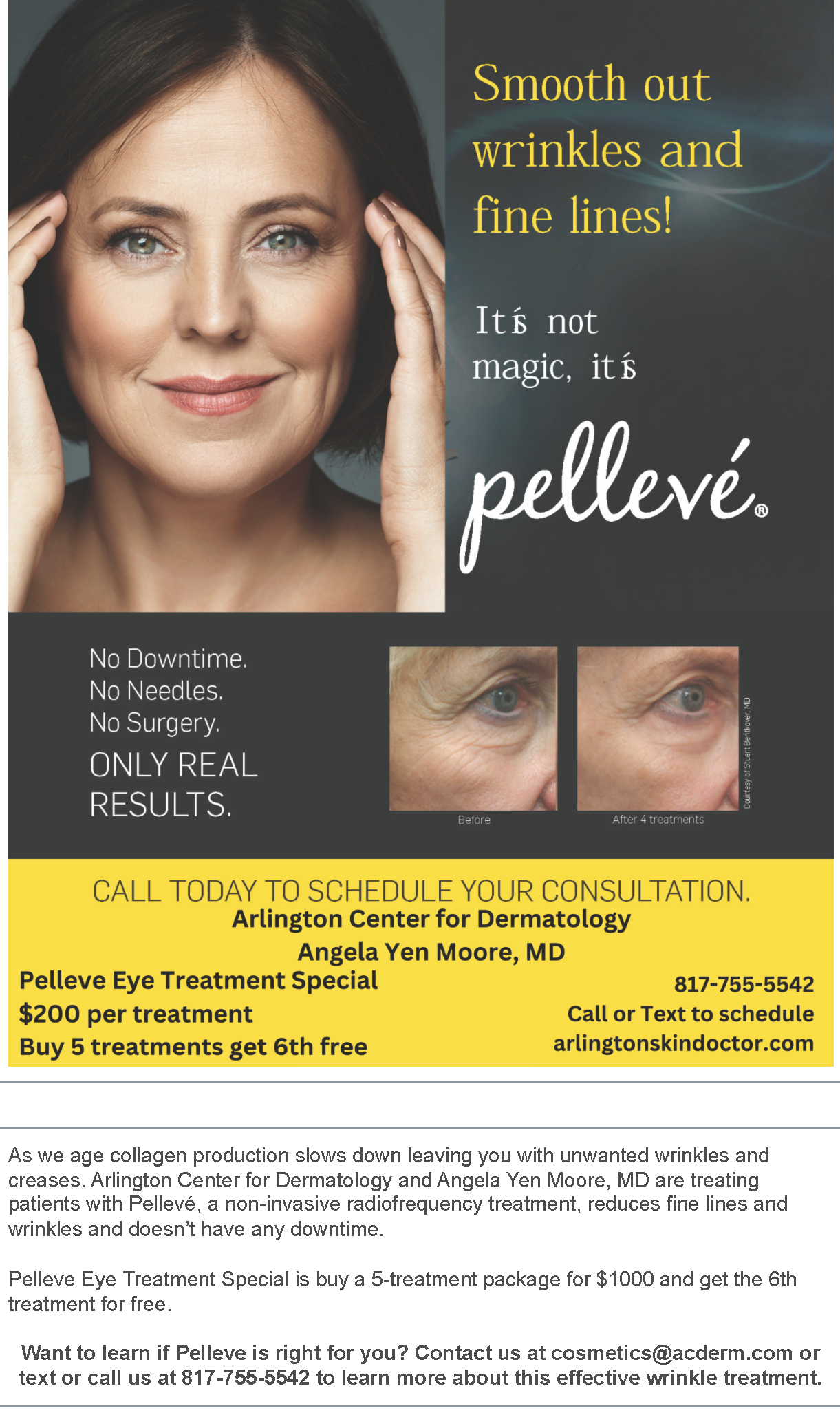
If you have hair loss you may be suffering from a condition called Alopecia. A new Topical cream study for ages 18 and older.
If interested, contact us at 817-755-5542 or email us at studies@acderm.com
Atopic Dermatitis Sleep Disturbances
New topica creaml study for 18 years and older.
Arlington Research Center is looking for adults, aged 18 years and up who have eczema and resulting sleep disturbances, to participate in a clinical research study evaluating an investigational cream.
How can you participate?
Inquire about how you can participate in this study today.
TO LEARN MORE ABOUT THE INTEGUMENT STUDY, CONTACT:
studies@acderm.com or call 817-795-7546 ext. 339 or Text 817-755-5542.
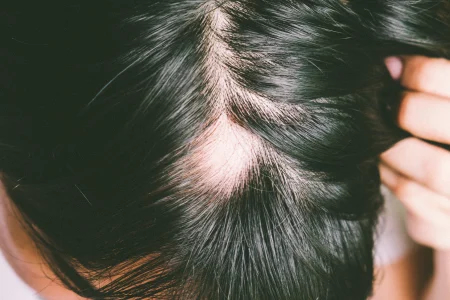
Hidradenitis Suppurativa – upcoming study
We know life with Hidradenitis Suppurativa isn’t always easy.
There are many unanswered questions surrounding the causes of HS and the painful and distressing symptoms it triggers in the body. With limited approved medical treatment options available, it’s important to keep looking for potential answers.
Call 817-795-7546 or email studies@acderm.com if you think you would like to participate in this study.
Arlington Research Center is looking for children and adults, aged 6 years and up who have eczema, to participate in a clinical research study evaluating an investigational cream. This study includes up to 5 visits for 2 months.
Studies for youth and adults. Must have a history of atopic dermatitis for at least 3 months for youth 6-17 years of age, and for at least 6 months for adults 18 years and older. Study patients previously on the ARQ-151 are excluded.
Also have a Atopic dermatitis study with an injection medication for patients 12 years of age and older
How can you participate?
Inquire about how you can participate in this study today.
TO LEARN MORE ABOUT THE INTEGUMENT STUDY, CONTACT:
studies@acderm.com or call 817-795-7546 ext. 339 or Text 817-755-5542.
New topical study for ages 2-12 years old. This study focuses on the scalp and body.
While severe psoriasis affects the skin, causing it to itch and burn, it can also have an emotional impact on adolescents, as well as their parents.
Because of the raised, red, scaly patches on their skin, many adolescents feel ashamed or embarrassed by their condition. These emotions can cause them to not want to hang out with friends, attend school functions, or participate in extracurricular activities.
For parents watching these physical and emotional effects, it’s difficult when other treatments have not been successful in managing their child’s psoriasis.
Because many adolescents and their families struggle with psoriasis, this research study is being conducted to evaluate an investigational drug for severe psoriasis. In this study, researchers want to learn more about the safety and effectiveness of the investigational drug.
The investigational drug has not been approved by any regulatory agency for the treatment of children and adolescents with this condition. It is only available to children and adolescents in research studies like this one.
The results of this study will provide more information about the investigational drug and whether it could one day be approved for children and adolescents with severe plaque psoriasis.
Who is eligible to participate in this study?
To be eligible for this study, patients must:
- Be 6 to 12 years of age
- Have been diagnosed with chronic plaque psoriasis
All study-related visits, tests, and drugs will be provided at no cost. In addition, compensation for study-related travel may be provided.